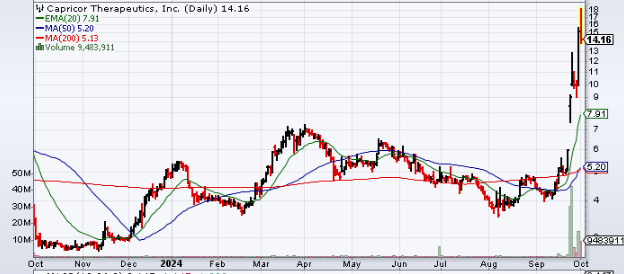
Capricor Therapeutics (NASDAQ:CAPR) Stock Surges After Intent to File Biologics License Application
The biotech firm Capricor Therapeutics (NASDAQ:CAPR), involved in the development of transformative exosome-based and cell therapies for tackling rare diseases, was on sharp focus yesterday. The company’s stock rallied strongly and delivered gains of 52% for the day amidst strong interest.
Capricor Therapeutics Announces Intent to File Biologics License Application for Full Approval of Deramiocel for the Treatment of Duchenne Muscular Dystrophy Cardiomyopathy
On September 24, Capricor Therapeutics hit the news cycle after it announced that, following a number of meetings with the United States Food and Drug Administration, the company was going to file a BLA (Biologics License Application). The BLA would be filed by the company on the basis of the natural history and existing cardiac data for deramiocel for the treatment of patients who suffered from DMD (Duchenne muscular dystrophy) cardiomyopathy.
The company announced last week that it was going to file the BLA at some point in October 2024 and seek complete approval for the treatment. The complete submission for the same would be made by the end of this year. In this context, it should be noted that currently there are no approved therapies for DMD cardiomyopathy.
In the news release, Capricor Therapeutics had also noted that on the basis of the data and the commitment of the FDA towards advancing these therapeutics, the company was also going to expand the scope of the treatment to skeletal muscle myopathy following the potential approval.
Key Quote
“There are currently no approved therapies for DMD cardiomyopathy, which is the leading cause of death in those with Duchenne. Based on the strength of our cardiac data, combined with the FDA’s commitment to advancing therapeutics for the treatment of rare diseases, we are seeking approval for the cardiomyopathy associated with DMD and will look to expand the label for skeletal muscle myopathy post-approval,” said Linda Marbán, Ph.D., Capricor’s chief executive officer. “This approach is the result of multiple in-depth meetings with the FDA where we showed robust and positive cardiac data from our HOPE-2 and HOPE-2 OLE studies compared to natural history data from a large cohort of patients.”
Fundamentals
| P/E Ratio | -16.35 |
| PEG Ratio | 13.28 |
| Price to Book | 42.29 |
| Price to Cash Flow | – |
| Price to Free Cash Flow | – |
| Total Sales (TTM) | 27.15 M |
| Revenue per Share (TTM) | 0.85 |
| Shares Outstanding | 32.539 M |
| Share Float (%) | 27.08 M (83.22%) |
| % Held by Institutions | 19.89 |
| Dividends Per Share (TTM) | – |
| Ex-Dividend Date | – |