BioVie Inc. (NASDAQ:BIVI) Stock More Than Doubled: What’s The Buzz?
If you are currently hunting for stocks that may have made strong gains last Friday, then it may be difficult to look past the BioVie Inc. (NASDAQ:BIVI) stock, which delivered gains of 110%. It is likely to be in focus among investors this morning.
BioVie Receives Notice of Allowance for Japan Patent Application Covering Novel Liquid Formulation of Terlipressin
The company hit the newswires on October 15 after BioVie announced that the Japan Patent Office had awarded it a Notice of Allowance for its application titled ‘Formulations of Terlipressin’. It was a major new development for the company since the Notice of Allowance would result in the awarding of the patent for the same in a matter of two to three weeks following the completion of all related administrative processes.
Key Details
The claims that had been allowed by the Japan Patent Office cover the unique liquid terlipressin acetate formulation with stability at room temperature for two years. The other claim is related to the fact that the product could be packaged inside a pre-filled syringe.
The claims also supported the company’s liquid formulation to be developed into a more patient-friendly ambulatory treatment for those suffering from hepatic failure and ascites in the United States. Considering the rally in the BioVie stock on Friday, it would be interesting to see if the stock can manage to hold on to its momentum this week.
Key Quote
“Terlipressin is a drug used in over 40 countries to treat related complications of liver cirrhosis that was only recently approved in the US as a lyophilized powder for in-hospital use, which requires reconstitution with sterile sodium chloride and refrigerated storage conditions for proper administration,” said Cuong Do, BioVie’s President and CEO. “Our novel liquid formulation of terlipressin is stable at room temperature for up to 24 months, offering an important advantage for treating patients with cirrhosis and ascites, particularly in the home-care setting. The prefilled syringe format can improve dosing convenience and safety. The receipt of this patent will significantly strengthen BIV201’s intellectual property protection and increase the value proposition behind our BIV201 program.”
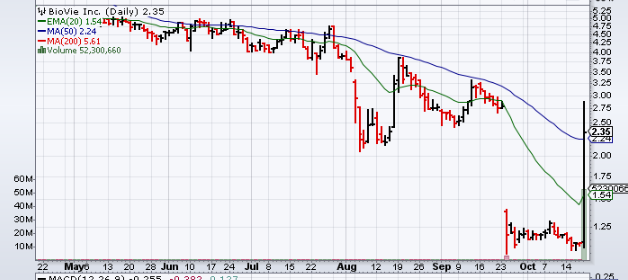
Traders Notes
| +/- EMA(20) | 1.54 (+52.60%) |
| +/- SMA(50) | 2.24 (+4.91%) |
| +/- SMA(200) | 5.61 (-58.11%) |
| 5-Day Perf. | +99.15% |
| 1-Month Perf. | -18.97% |
| 3-Month Perf. | -43.02% |
| 6-Month Perf. | -51.88% |
| YTD Perf. | -81.35% |
| 1-Year Perf. | -92.59% |
| RSI(14) | 64.53 |
| ATR(14) | 0.28 |
| ADX(14) | 41.08 |

