Sangamo Therapeutics Inc. (Nasdaq: SGMO) Stock Soars 50% This Week: What’s The Buzz?
There are some companies that are expected to come into focus today following the performance from Tuesday, and one of those is Sangamo Therapeutics Inc. (Nasdaq: SGMO), which saw its stock record 32% gains amidst strong interest.
Regulatory Breakthrough
The breakout rally in the stock was triggered yesterday after the company provided the potential outcome from the successful meetings with the United States Food and Drug Administration. Sangamo Therapeutics stated that the meetings provided a pathway by way of which it could get accelerated approval for the product ST 920. The product in question is the company’s gene therapy product meant for the treatment of those suffering from Fabry disease.
Key Takeaways
In the news release, Sangamo Therapeutics announced that the United States FDA agreed that in a Type B interaction, the data gleamed from the Phase ½ STAAR study could act as the basis for approval under the provisions of the Accelerated Approval Program. It was also noted that the complete data set that would be necessary for the Accelerated Approval pathway would be made available at some point in the first six months of 2025.
The company stated that the situation could open up the opportunity for a BLA submission in the latter half of 2025. It could be a good time to consider adding the Sangamo Therapeutics stock to your watch lists.
CEO Quote
“Fabry is a debilitating disease, for which there is a serious unmet medical need,” said Sandy Macrae, Chief Executive Officer of Sangamo. “I strongly believe in the potential for ST-920 to alleviate many manifestations of Fabry disease and am delighted to have a clear regulatory pathway that could bring this treatment to patients significantly sooner than originally anticipated.”.
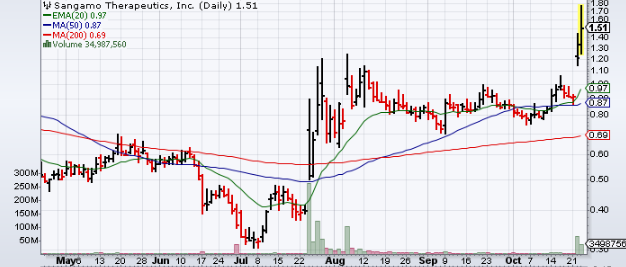
Technicals
| +/- EMA(20) | 0.97 (+55.67%) |
| +/- SMA(50) | 0.87 (+73.56%) |
| +/- SMA(200) | 0.69 (+118.84%) |
| 5-Day Perf. | +52.22% |
| 1-Month Perf. | +65.03% |
| 3-Month Perf. | +276.28% |
| 6-Month Perf. | +189.88% |
| YTD Perf. | +177.93% |
| 1-Year Perf. | +210.51% |
| RSI(14) | 82.36 |
| ATR(14) | 0.15 |
| ADX(14) | 27.46 |
| Beta (5Y) | 1.30 |

