Capricor Therapeutics Inc. (NASDAQ:CAPR) Stock Rockets 53% On Heavy Volume: How To Trade Now?
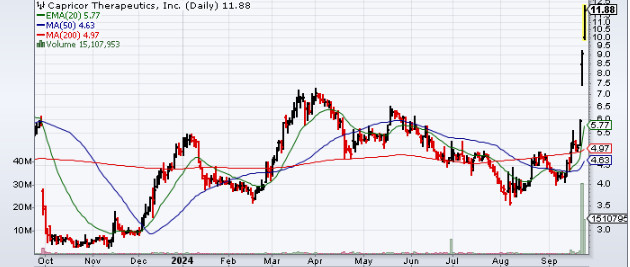
If you are currently on the lookout for biotech stocks to follow, then Capricor Therapeutics Inc. (NASDAQ:CAPR) could be the one to add to your watch list. Yesterday the stock was in sharp focus and ended up with gains of as much as 53% amidst heavy trading.
Capricor Therapeutics Announces Intent to File Biologics License Application for Full Approval of Deramiocel for the Treatment of Duchenne Muscular Dystrophy Cardiomyopathy
Biotech is involved in the development of innovative cell- and exosome-based therapeutics for treating rare diseases. The company announced yesterday that after having had meetings with the United States Food and Drug Administration, it had decided to file a BLA (Biologics License Application).
The BLA would be based on the existing natural history and cardiac data related to deramiocel for the purpose of treating patients suffering from DMD (Duchenne molecular dystrophy) cardiomyopathy. The Chief Executive Officer of Capricor Therapeutics, Linda Marban, spoke about the latest developments yesterday.
Marban noted that there were no approved therapies for the treatment of DMD cardiomyopathy. She went on to note that on the basis of the strong cardiac data at the company’s disposal and the commitment of the FDA in promoting therapeutics meant for treating rare diseases, Capricor Therapeutics was going to look for approval of its own product for treating the condition.
Key Quote
“There are currently no approved therapies for DMD cardiomyopathy, which is the leading cause of death in those with Duchenne. Based on the strength of our cardiac data, combined with the FDA’s commitment to advancing therapeutics for the treatment of rare diseases, we are seeking approval for the cardiomyopathy associated with DMD and will look to expand the label for skeletal muscle myopathy post-approval,” said Linda Marbán, Ph.D., Capricor’s chief executive officer. “This approach is the result of multiple in-depth meetings with the FDA where we showed robust and positive cardiac data from our HOPE-2 and HOPE-2 OLE studies compared to natural history data from a large cohort of patients.”