NovAccess Global (OTCMKTS:XSNX) Stock Rockets On Filing of Orphan Drug Application for TLR-AD1
Biomedical firm, NovAccess Global (OTCMKTS:XSNX), develops novel immunotherapies for brain tumor patients. The ultimate goal is to discover, develop and bring to market novel and innovative medicine and medical devices to improve the quality of care for cancer and neurological patients. At present, it is developing a cancer vaccine therapy that boosts the patient’s immune response against brain tumors.
Market Stats
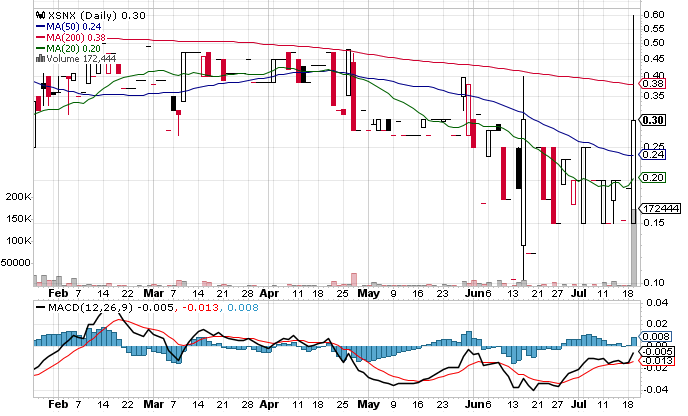
On Tuesday, XSNX stock soared 57% to $0.2980 with 172K shares, compared to its average volume of 6K shares. The stock moved within a range of $0.1500 – 0.6000 after opening trading at $0.15.
CORRECTION: NovAccess Global Announces Filing of Orphan Drug Application for TLR-AD1
NovAccess Global Inc. has announced that it has filed an application with the U.S. Food and Drug Administration (FDA) to receive Orphan Drug Designation (ODD) for TLR-AD1. This is vaccine immunotherapy aimed at treating aggressive brain cancers, including glioblastoma and other high-grade gliomas. The FDA’s Office of Orphan Products Development grants orphan designation status to investigational drugs and therapies addressing rare medical diseases or conditions that affect fewer than 200,000 people in the United States.
Dr. Christopher Wheeler, President of StemVax Therapeutics (a wholly-owned subsidiary of NovAccess Global), has expressed his elation at the filing of an Orphan Drug application for TLR-AD1. If they are successful in getting FDA approval, it would give them special status, and allow the speeding up of the development of therapies to treat glioblastoma patients.
The ODD process supports the development and evaluation of new treatments for rare diseases which is a key priority for both the FDA and for NovAccess Global. Receiving this important designation would be a turning point in the development of TLR-AD1 and highlight the need for potential new treatment options for patients with aggressive brain cancers. There is no available immunotherapy treatment yet, leaving only the option of highly invasive and complicated surgery.
Glioblastoma is a form of aggressive brain cancer that annually impacts approximately 250,000 people globally. According to NovAccess scientists and published reports, the number is rising alarmingly in several countries. As per market data, glioblastoma is responsible for approximately 50% of all malignant brain cancers diagnosed in the United States each year, and more than 10,000 Americans die from this tumor type annually. Less than 5% of people with this cancer live longer than 5 years after their diagnosis.
The global glioblastoma treatment market was estimated to be valued in excess of $2 billion in 2020, with projections for a compounded annual growth rate of more than 8% throughout the remainder of the decade. Dr. Wheeler further elaborated that upon receiving ODD, the product will considerably bolster NovAccess Global’s intellectual property portfolio, which presently includes the rights to U.S. patent #US9764014B2 granted under the “Cancer Antigens” category. It falls under the “treatment of cancer using vaccination therapy.” As of now, they are happy with the progress being made toward building a platform for novel immunotherapy for brain tumor patients.
Key Quote
“We are very excited to announce the filing of an Orphan Drug application for TLR-AD1. If we obtain FDA approval it would grant us special status and enable the acceleration of the development of our therapies to treat glioblastoma patients,” Dr. Christopher Wheeler, President of StemVax Therapeutics. “The ODD process supports the development and evaluation of new treatments for rare diseases which is a key priority for both the FDA and for NovAccess Global. Receiving this important designation would represent a milestone in the development of TLR-AD1 and would highlight the need for potential new treatment options for patients with aggressive brain cancers which today have no immunotherapy treatment leaving only the option of highly invasive and complicated surgery.”
Traders Corner
XSNX stock is trading above the 20-Day and 50-Day Moving averages of $0.0242 and $0.2010. However, the stock is trading below the 200-Day moving average of $0.3991.

