Tevogen Bio (Nasdaq: TVGN) Stock Recovers After Major Updates
On Thursday, Tevogen Bio (Nasdaq: TVGN) was in significant focus among investors, and the stock climbed by 23% after the company reissued the top-line results from the TVGN 489 proof-of-concept clinical trial. The trial had been commissioned to check the feasibility and safety of the product.
Tevogen Bio Highlights Promising Trial Data as CEO Leads Patient Advocacy at Capitol Hill, Pledging to Leverage Multi-Billion Dollar Company Assets to Combat Long COVID and Spearhead a New Wave of Medical Innovation
It was a major new update from the company since TVGN 489 was the first clinical product that had been developed through the deployment of Tevogen Bio’s self-developed ExacTcell Technology. The product had been developed to treat patients at high risk of SARS-CoV-2, and the latest announcement clearly led to significant excitement in the markets.
The Trial
The open-label comparative trial for the product had been done at the Philadelphia-based Thomas Jefferson University Hospital to figure out the safety and feasibility of TVGN 489. A total of 12 high-risk patients infected with SARS-CoV-2 were treated as part of the trial.
Additionally, 18 other patients were also part of the study who had not been treated with TGVN 489 and had been part of the observational portion of the study. Considering the latest announcement from Tevogen Bio, it may be a good idea for investors to add the stock to their watch lists.
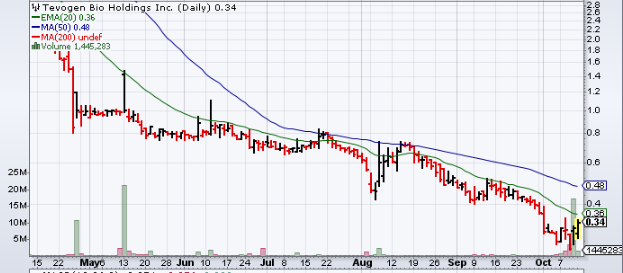
Traders Notes
| +/- EMA(20) | 0.37 (-8.92%) |
| +/- SMA(50) | 0.48 (-29.79%) |
| +/- SMA(200) | 1.87 (-81.98%) |
| 5-Day Perf. | +20.79% |
| 1-Month Perf. | -23.22% |
| 3-Month Perf. | -51.85% |
| 6-Month Perf. | -86.36% |
| YTD Perf. | – |
| 1-Year Perf. | – |
| RSI(14) | 42.3 |
| ATR(14) | 0.05 |
| ADX(14) | 32.02 |
| Beta (5Y) | 1.97 |

